9. Alkenes and Alkynes
Alkene Stability
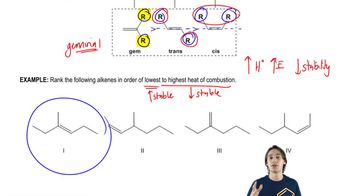
Problem 7a
Textbook Question
Textbook QuestionThe energy difference between cis- and trans-but-2-ene is about 4 kJ/mol; however, the trans isomer of 4,4-dimethylpent-2-ene is nearly 16 kJ/mol more stable than the cis isomer. Explain this large difference.
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
5mPlay a video:
442
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 2 videos