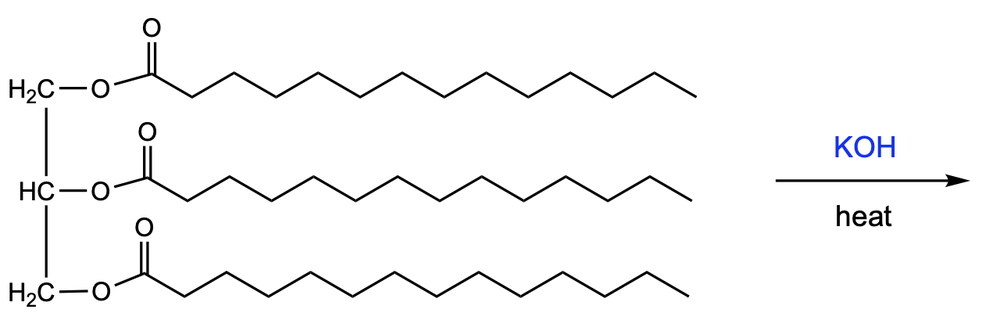
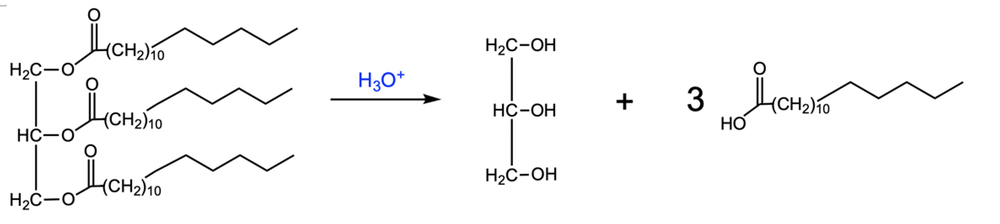
Saponification is a chemical process that involves the base-catalyzed hydrolysis of esters, specifically triglycerides, to produce glycerol and fatty acid salts. In this reaction, hydroxide ions (OH-) break the ester bonds, resulting in the formation of carboxylate anions instead of carboxylic acids. This is significant because the negatively charged oxygen atoms in the carboxylate anions bond with positively charged sodium ions (Na+) when sodium hydroxide (NaOH) is used as the base, leading to the creation of salts of fatty acids.
The process of saponification is crucial in the production of soaps. When sodium hydroxide is employed, the outcome is solid soap, while the use of potassium hydroxide (KOH) yields liquid soap. This distinction is important for various applications in the soap-making industry. Overall, saponification not only illustrates the principles of ester hydrolysis but also highlights its practical applications in everyday products like soaps.