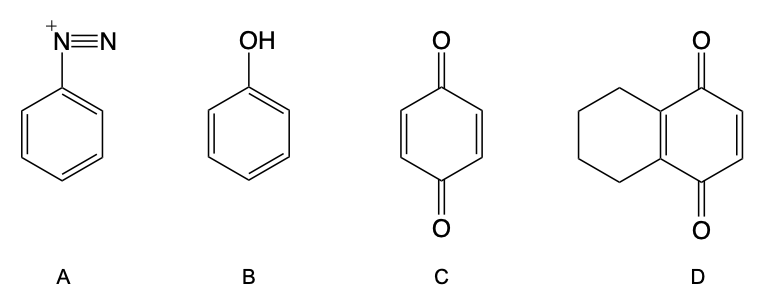
The oxidation of phenol to quinone is an important chemical transformation that occurs in the presence of strong oxidizing agents, such as dichromate ions (\( \text{Cr}_2\text{O}_7^{2-} \)). This reaction highlights the unique properties of phenol compared to other alcohols, particularly tertiary alcohols, which do not undergo oxidation due to the absence of alpha hydrogens on the carbon bearing the hydroxyl group.
Quinone, specifically 1,4-benzoquinone, is characterized by its conjugated six-membered ring structure, which contains two carbonyl groups positioned 1,4 to each other. The nomenclature of quinone can be broken down as follows: "cyclo" indicates a ring structure, "hexa" refers to the six carbon atoms in the ring, "diene" signifies the presence of two double bonds, and "dione" denotes the two carbonyl groups. Thus, 1,4-benzoquinone can also be referred to as cyclohexadienedione.
In contrast to tertiary alcohols, which do not react due to the lack of reactive hydrogens, phenol can be oxidized effectively to form quinone. The resulting quinone features a conjugated system with alternating double bonds and carbonyls, contributing to its stability and reactivity. This transformation is significant in organic chemistry, showcasing the distinct reactivity of phenolic compounds.