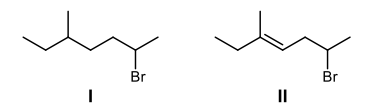
Reactions at the allylic position involve the carbon atom adjacent to a double bond, known as the allylic carbon. In a molecular structure featuring double-bonded carbons, these carbons are referred to as the vinylic carbons, while the allylic position is the carbon next to them. Understanding the behavior of allylic compounds is crucial, as they exhibit distinct reactivity compared to alkenes.
In the context of nucleophilic substitution and elimination reactions, allylic compounds demonstrate faster reaction rates than their alkyl counterparts in SN1 and E1 reactions. Specifically, allylic halides react more rapidly than alkyl halides in SN2 and E2 reactions. This increased reactivity can be attributed to the stability provided by resonance in allylic systems.
When considering radical reactions, substitution occurs preferentially at the allylic position, highlighting the unique reactivity of these compounds. Additionally, allylic anions are notable for their rapid rearrangement due to resonance stabilization, which allows for the delocalization of negative charge across the molecule.
Overall, the allylic position plays a significant role in determining the pathways and mechanisms of various chemical reactions, making it an essential concept in organic chemistry.