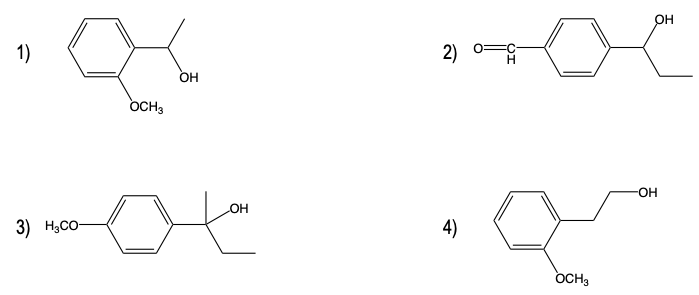
In organic chemistry, the benzylic position refers to the carbon atom directly attached to a benzene ring. This position is particularly reactive due to the stability of the reaction intermediates formed during various chemical reactions. The reactivity at the benzylic position is largely attributed to resonance effects, which allow for the delocalization of electrons, enhancing the stability of carbocations and other intermediates.
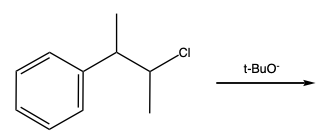
When discussing substitution and elimination reactions at the benzylic position, it is essential to understand the mechanisms involved. The SN1 and E1 mechanisms involve the formation of a carbocation intermediate. In these reactions, the first step is the ionization of the benzylic halide to form a stable carbocation, which can then undergo either nucleophilic substitution or elimination.
Conversely, the SN2 and E2 mechanisms involve a concerted process where the nucleophile attacks the benzylic carbon while the leaving group departs simultaneously. This mechanism is characterized by a single transition state and does not involve the formation of a stable carbocation.
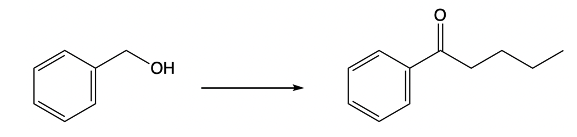
Additionally, the oxidation of benzylic alcohols is another important reaction to consider. Benzylic alcohols can be oxidized to form benzylic aldehydes or ketones, depending on the conditions used. This transformation is significant in synthetic organic chemistry, as it allows for the functionalization of the benzylic position.
Overall, the unique properties of the benzylic position, including its ability to stabilize intermediates through resonance, make it a focal point for various substitution and elimination reactions in organic synthesis.