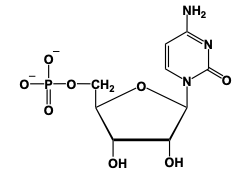
Nucleosides are named based on the nitrogenous base they contain, with specific suffix modifiers indicating their classification. Nitrogenous bases are categorized into two groups: pyrimidines and purines. Pyrimidines, which consist of a single ring structure, have their names modified with the suffix idine, while purines, characterized by a double ring structure, use the suffix osine.
For example, in RNA, the nitrogenous base uracil combines with ribose sugar through a condensation reaction, resulting in the formation of a glycosidic bond between the nitrogen atom of uracil and the anomeric carbon of ribose. This process leads to the creation of the nucleoside uridine, where the suffix of uracil is changed to idine.
In contrast, DNA utilizes deoxyribose sugar instead of ribose. When adenine, a purine, forms a nucleoside, it also undergoes a condensation reaction to create a glycosidic bond. However, since DNA's sugar lacks a hydroxyl group at the second carbon, the prefix deoxy is added to the name. Thus, adenine becomes deoxyadenosine, reflecting both the absence of the hydroxyl group and the purine structure.
Understanding these naming conventions is crucial for accurately identifying and differentiating between various nucleosides in RNA and DNA.