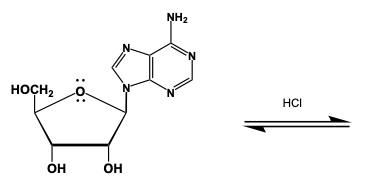
Nucleosides, which consist of a sugar and a nitrogenous base, are generally resistant to hydrolysis. However, under strong acidic conditions and with sufficient time, they can undergo hydrolysis, resulting in the formation of free sugar and a nitrogenous base. The mechanism of acid-catalyzed hydrolysis of nucleosides can be delineated into four key steps.
In the first step, a proton transfer occurs, which activates the nucleoside for further reaction. The second step involves the loss of the leaving group, which is crucial for the subsequent nucleophilic attack. In the third step, a nucleophile attacks the activated nucleoside, leading to the formation of the products. Finally, the fourth step involves another proton transfer, which helps stabilize the resulting products.
Understanding these steps is essential for grasping the overall mechanism of nucleoside hydrolysis, as each step plays a vital role in the transformation process. This knowledge is foundational for further studies in biochemistry and molecular biology, particularly in the context of nucleic acid metabolism and enzymatic reactions.