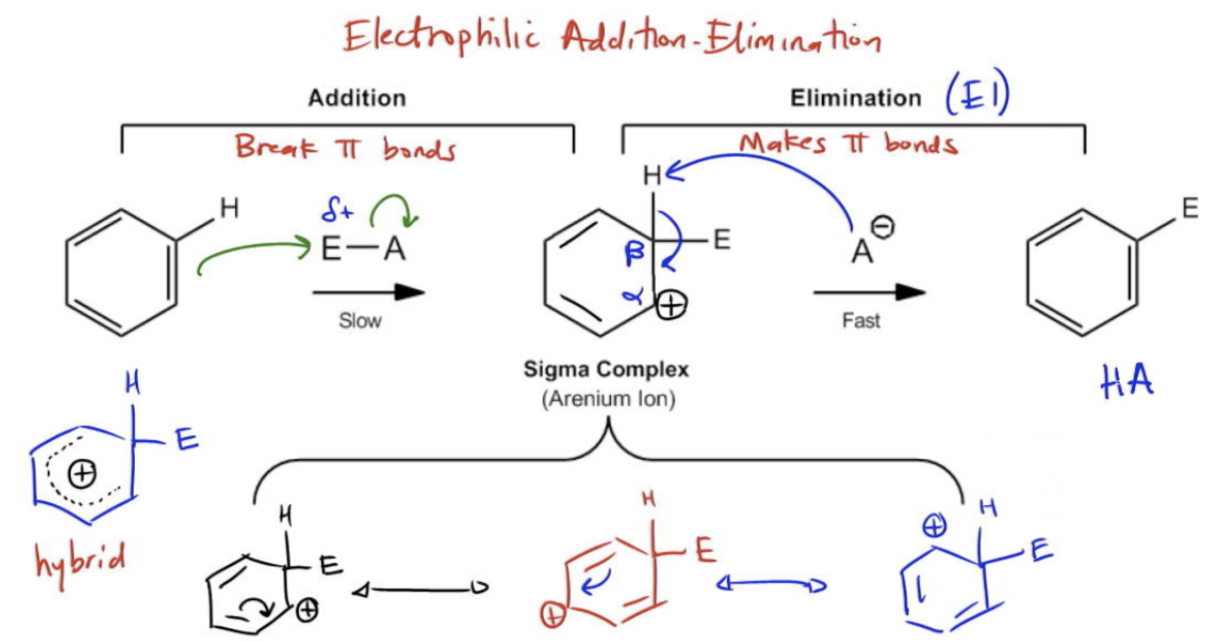
The first thing that should strike us when we look at this mechanism is that it's a 2 step mechanism, and it can be summarized by these words: addition and elimination. These are the two stages of an EAS mechanism. Now, remember that an addition reaction is one that breaks pi bonds. Typically, you break a pi bond and you add 2 sigma bonds. Remember that an elimination is the opposite. An elimination reaction makes pi bonds. That makes sense because, remember, we said that at the end, we want aromatic C to be restored. What we're saying is that we're going to break that double bond for a little bit, but at the end, we're going to make it again. We're going to get that aromatic product. For that reason, this mechanism is also known as the electrophilic addition elimination mechanism. That just explicitly says the 2 steps of EAS. There's an addition elimination, addition first elimination, and the whole thing happens through an electrophilic reagent. Let's go ahead and take a look at this first step and see if you guys can theorize where do you think this arrow would first come from. Now, this molecule EA just means that it's some electrophile, so some kind of very strong partial positive or positive attached to some conjugate that we'll use later. Since we have a positive there, you know that the arrows are always going to start from the most negatively charged thing. And for EAS, that's always the benzene. The benzene has tons of electrons, so even more so than a typical double bond, this thing's got loads of electrons that it can use to attack the electrophile. Let's go ahead and draw that. We're going to attack our electrophile, and if we make that bond, we have to break a bond. So we're going to break our bond to the A, forming the conjugate base, which is going to be the conjugate for this reaction. Now what we're going to form is this intermediate. And this intermediate is called the sigma complex, or it's also called an arenium ion. This is one of the most important intermediates of organic chemistry too, so I definitely want you guys to really understand it. What happens is that 2 of the double bonds stay exactly the same. 1, 2, like that. But now we've got the electrophile adding to one side and a cation on the other because we're missing a bond. We just broke the pi bond. Nothing else came to replace it. We've got literally a missing bond at that site. Now, one of the things that stabilizes this sigma complex is the fact that there's tons of resonance possible. So you might be asked to draw the resonance structures of this complex. Let's go ahead and do that now. We're just going to do that underneath here. Let's bring the top structure down. And let's go ahead and just draw the first one exactly the way that I drew it at the top, and then we'll resonate it. That's our first resonance structure of the sigma complex. The next structure would have the double bond moving to take its spot. Remember that cations can always move with one arrow, so we would swing this door like a door hinge, and we would make the next resonance structure. The next resonance structure is in the same; all the atoms are the same, but now we have our cation over here. Now we're going to move this pi bond one more time, and we're going to get the last resonance structure of my arenium ion or sigma complex. As you guys can imagine, this cation is much less stable than an aromatic compound. But it is stabilized by the fact that it can resonate 3 times. So it's distributed across all 5 of those carbons. As you guys might guess, the resonance hybrid would be drawn simply by drawing a dotted line around all 5 of those and adding a positive. That would be the way that we would represent our sigma complex hybrid. Okay. Awesome. So this sigma complex, even though it's stabilized by resonance, it's still the highest, you know, it's still much higher energy on my energy diagram than the reactants or the products. So this is going to be my slow step because it's difficult to make this intermediate. So now what do we do? Now we need an elimination step. Remember that elimination makes double bonds. The way that's going to work is that's where my conjugate base comes in. My conjugate base is going to come in and do an elimination reaction, basically like an E1. This is essentially going to be an E1 mechanism if you recall back to organic chemistry 1. You've got a carbocation, and we're going to go ahead, and we're going to take out, do a beta elimination. If this is my alpha carbon, then this is my beta carbon. We're going to do a beta elimination on a hydrogen and reform the double bond. This step is the fast step because it's easy to eliminate because we're making an aromatic compound. The slow step or the rate-determining step of this reaction is how quickly we can make that sigma complex. As you guys see, what are we going to have at the end? We're going to have now my substituted arene, and we're going to have some kind of acid. But what we're really concerned about and what your professor is really concerned with is the actual benzene ring, the actual aromatic product. That pretty much does it for this mechanism. Now we're going to spend some time talking about the specific electrophiles that we're going to use because this mechanism just used E. Now this mechanism is going to apply for all the different electrophiles we learn in this chapter, but it's going to be your job to know exactly which electrophiles we can use. Let's go ahead and learn about the electrophiles of electrophilic aromatic substitution.