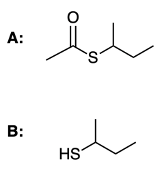
In the study of organic chemistry, the hydrolysis of thioesters is an important reaction to understand. Thioesters, which are sulfur analogs of regular esters, undergo acid-catalyzed hydrolysis to yield a carboxylic acid and a thiol. This reaction is characterized by a nucleophilic acyl substitution (NAS) mechanism, which consists of several key steps.
The first step involves protonation, where a proton (H+) is added to the thioester, enhancing its electrophilicity. In the second step, a nucleophile attacks the carbonyl carbon of the thioester, forming a tetrahedral intermediate. This is followed by a proton transfer in the third step, which facilitates the rearrangement of the intermediate. The fourth step involves the departure of the leaving group, which in this case is the thiolate ion. Finally, the fifth step is deprotonation, restoring the neutral charge and completing the formation of the products.
It is essential to note that steps one, three, and five all involve the movement of protons, highlighting the significance of proton transfer in the mechanism. Understanding this sequence of events is crucial for mastering the hydrolysis of thioesters and recognizing the broader implications of nucleophilic acyl substitution reactions in organic synthesis.