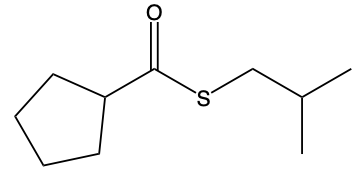
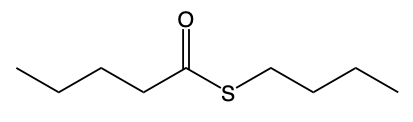
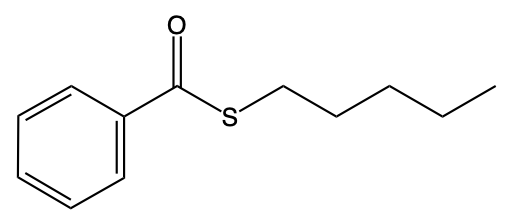
Thioesters are important compounds in organic chemistry, serving as sulfur analogs of esters. They can be categorized into two main types: S-alkyl thioesters and O-alkyl thioesters. S-alkyl thioesters, the more common variant, feature a carbonyl group (C=O) that is bonded to a sulfur atom (S), which is further connected to an R group, typically representing a carbon group. This structure is crucial for understanding the reactivity and properties of thioesters.
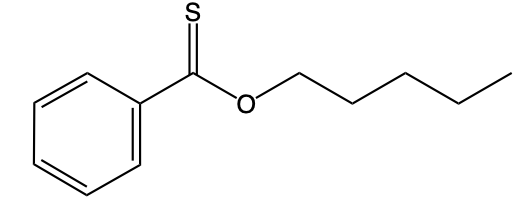
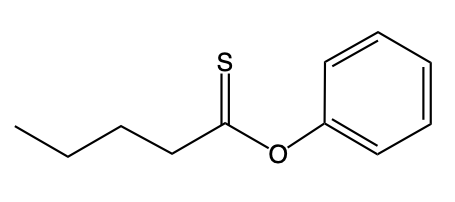
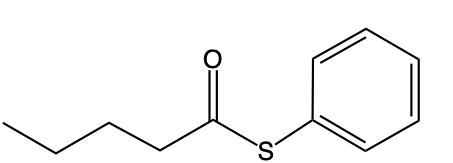
On the other hand, O-alkyl thioesters differ significantly in their structure. Instead of the traditional carbonyl group bonded to oxygen, they possess a carbon atom double-bonded to sulfur (C=S). This carbon is also single-bonded to an oxygen atom (O), which is then connected to an R group. The distinction between these two types of thioesters is essential for their chemical behavior and applications in various biochemical processes.
In summary, thioesters can exist as either S-alkyl thioesters or O-alkyl thioesters, with S-alkyl thioesters being the more prevalent form. Understanding these structures is fundamental for exploring their roles in organic reactions and biological systems.