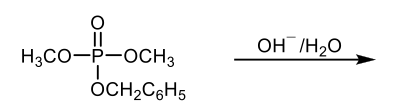Phosphate esters are important compounds formed by the addition of alkyl groups to a phosphate ion, which has the chemical formula \( \text{PO}_4^{3-} \). The structure of the phosphate ion features a phosphorus atom at the center, double-bonded to one oxygen atom and single-bonded to three others, allowing for resonance structures that contribute to its stability.
Phosphate esters can be categorized based on the number of alkyl groups (denoted as R groups) attached to the negatively charged oxygens of the phosphate ion. When one R group is present, the compound is referred to as a monoester. If there are two R groups, it is called a diester, and with three R groups, it is classified as a triester. The R groups can either be identical or different, leading to a variety of structural possibilities.
The reactivity of phosphate esters towards hydrolysis is influenced by the overall charge of the molecule. As the number of R groups increases, the charge on the phosphate decreases, enhancing the reactivity. Consequently, monoesters are the least reactive, followed by diesters, with triesters being the most reactive due to their lower overall charge. This trend highlights the significance of substitution on the negatively charged oxygens, which plays a crucial role in the chemical behavior of these esters.