The ψ2 molecular orbital for octa-1,3,5,7-tetraene is shown. Draw ψ3.
<IMAGE>

 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem: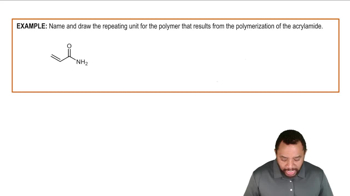


 5:29m
5:29mMaster Definition of Conjugation with a bite sized video explanation from Johnny
Start learning