Textbook Question
Potassium, a silvery metal, reacts with bromine, a corrosive, reddish liquid, to yield potassium bromide, a white solid. Write the balanced equation, and identify the oxidizing and reducing agents.
1920
views

 Verified step by step guidance
Verified step by step guidance
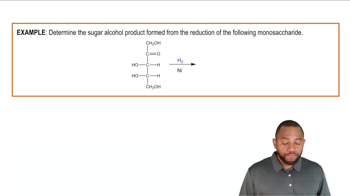

Potassium, a silvery metal, reacts with bromine, a corrosive, reddish liquid, to yield potassium bromide, a white solid. Write the balanced equation, and identify the oxidizing and reducing agents.
Assume that the mixture of substances in drawing (a) undergoes a reaction. Which of the drawings (b)–(d) represent a product mixture consistent with the law of conservation of mass?
Reaction of A (green spheres) with B (blue spheres) is shown in the following diagram:
Which equation best describes the reaction?
a. A2 + 2 B → A2B2
b. 10 A + 5 B2 → 5 A2B2
c. 2 A + B2 → A2B2
d. 5 A + 5 B2 → 5 A2B2