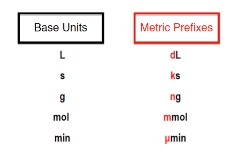
Understanding metric prefixes is essential for performing calculations in chemistry, as they serve as modifiers that represent multiples of ten. The metric prefix multipliers range from \(10^{12}\) to \(10^{-12}\), which is typically sufficient for introductory chemistry courses. For higher-level studies, prefixes such as \(10^{-15}\) and \(10^{15}\) may be encountered.
Here’s a breakdown of the common metric prefixes:
- Terra (T): \(10^{12}\)
- Giga (G): \(10^{9}\)
- Mega (M): \(10^{6}\)
- Kilo (k): \(10^{3}\) (as in kilometer)
- Hecto (h): \(10^{2}\)
- Deka (da): \(10^{1}\)
- Base Unit: (e.g., liters, seconds)
- Deci (d): \(10^{-1}\)
- Centi (c): \(10^{-2}\) (as in centimeter)
- Milli (m): \(10^{-3}\) (as in milliliters)
- Micro (µ): \(10^{-6}\)
- Nano (n): \(10^{-9}\)
- Pico (p): \(10^{-12}\)
To aid in memorization, a mnemonic device can be employed: "The great monarch, King Henry's daughter Barbara, drinks chocolate milk until 9 PM." Each initial corresponds to a metric prefix, helping to recall their order from \(10^{12}\) down to \(10^{-12}\).
As you study metric prefixes, remember to consult with your professor to confirm the specific range you need to master for your course. This foundational knowledge will be crucial as you apply these prefixes to base units in future calculations.