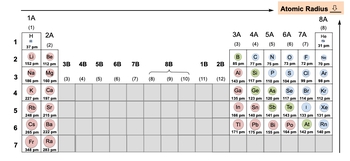
Atomic radius refers to the distance from an atom's nucleus to its outermost electron shell, known as the valence shell. The nucleus, located at the center, contains protons, which are positively charged, and neutrons, which are neutral. The atomic radius, denoted as r, is crucial for understanding the size of an atom.
As we move down a group in the periodic table, the atomic radius increases. This is because additional electron shells are added, allowing the atom to accommodate more electrons. Each new shell increases the distance between the nucleus and the outermost electrons, resulting in a larger atomic radius.
Conversely, when moving across a period from left to right, the atomic radius tends to decrease. This occurs because, although the number of electrons increases, they are added to the same shell. The increased number of electrons within that shell enhances the attraction between the electrons and the nucleus, effectively pulling the outer electrons closer and reducing the atomic radius.
In summary, the periodic trend for atomic radius shows an increase as you move down a group due to the addition of electron shells, while it decreases across a period as the effective nuclear charge increases, drawing electrons closer to the nucleus. This dual behavior highlights the complex interactions between electron shells and nuclear charge in determining atomic size.