Ions are formed when elements either lose or gain electrons, aiming to achieve a stable electron configuration similar to that of noble gases. Metals typically lose electrons, resulting in positively charged ions known as cations, while nonmetals tend to gain electrons, forming negatively charged ions called anions. This process of electron transfer is driven by the desire for stability in the electron arrangement.
Additionally, the term isoelectronic refers to different elements or ions that possess the same number of electrons. Understanding this concept is crucial when studying ionic formation, as it highlights the relationship between electron configuration and the stability of ions. In summary, the formation of ions through the gaining or losing of electrons is a fundamental concept in chemistry that explains how elements achieve a noble gas-like stability.

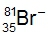 .
.




