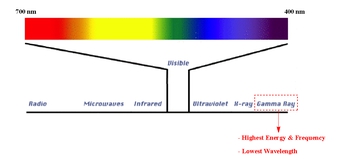
Gamma radiation is a form of electromagnetic radiation that occupies a position at the high-energy end of the electromagnetic spectrum. It is characterized by having the highest energy, the shortest wavelength, and the highest frequency among all types of electromagnetic waves. Understanding the relationship between energy, frequency, and wavelength is crucial in grasping the properties of gamma rays.
In the context of the electromagnetic spectrum, energy and frequency are directly proportional. This means that as the energy of a wave increases, its frequency also increases. Conversely, wavelength is inversely proportional to both energy and frequency. Therefore, when energy and frequency are high, the wavelength is low, and vice versa. Wavelength is defined as the distance between consecutive crests of a wave, while frequency refers to the number of wave cycles that pass a given point in one second.
For gamma rays, their high energy translates to a very high frequency and a correspondingly short wavelength. While cosmic rays possess even higher energy levels than gamma rays, they are not typically covered in basic chemistry discussions. Thus, within the scope of general chemistry, gamma rays are recognized as having the highest energy, highest frequency, and shortest wavelength in the electromagnetic spectrum.