Density is defined as the mass of an object divided by its volume, expressed mathematically as:
D = \frac{m}{V}
where D is density, m is mass, and V is volume. This concept can be applied to various geometric objects, each with its own volume formula.
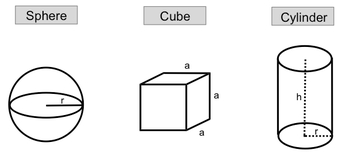
For a sphere, the volume can be calculated using the formula:
V = \frac{4}{3} \pi r^3
Here, r represents the radius, which is the distance from the center of the sphere to its surface. Understanding this formula allows you to determine the volume of a sphere when the radius is known.
In the case of a cube, where all sides are equal in length, the volume is given by:
V = a^3
In this formula, a denotes the length of one edge of the cube. This straightforward relationship makes it easy to calculate the volume of a cube based on the length of its sides.
For a cylinder, the volume is determined by both the radius and the height, expressed as:
V = \pi r^2 h
In this equation, r is the radius of the base of the cylinder, and h is the height. This formula highlights the importance of both dimensions in calculating the volume of a cylinder.
Once the volume of these geometric shapes is established, you can relate it back to density by rearranging the density formula to find mass or volume as needed. This foundational understanding of density and volume is crucial for solving various physics and engineering problems involving geometric objects.