8. Gases, Liquids and Solids
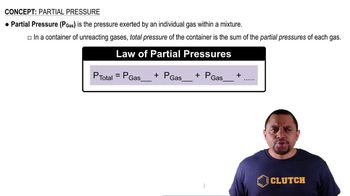
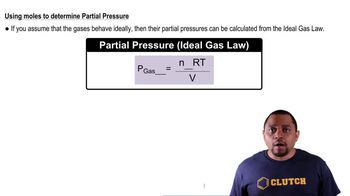
Dalton's Law: Partial Pressure (Simplified)
Problem 78
Textbook Question
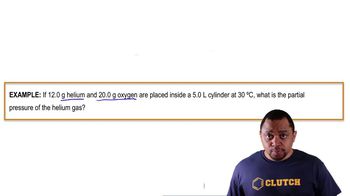
Textbook QuestionA mixture of nitrogen (N₂) and helium has a volume of 250 mL at 30 °C and a total pressure of 745 mmHg. (8.5, 8.6, 8.7) a. If the partial pressure of helium is 32 mmHg, what is the partial pressure of the nitrogen?
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
2mPlay a video:
176
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 6 videos