Identify the reactant that is a Brønsted–Lowry acid and the reactant that is a Brønsted–Lowry base in each of the following:
a. HI(aq) + H2O(l) → I-(aq) + H3O+(aq)
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem: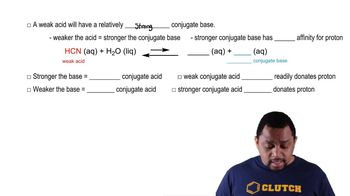



 2:08m
2:08mMaster Bronsted-Lowry Acid-Base Theory with a bite sized video explanation from Jules
Start learning