Textbook Question
One of the ions of tin is tin(IV).
b. How many protons and electrons are in the ion?
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem:

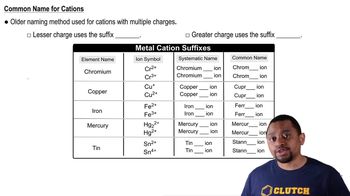

 1:24m
1:24mMaster Naming Monoatomic Cations Concept 1 with a bite sized video explanation from Jules
Start learning