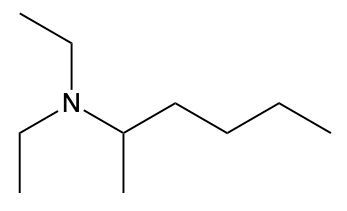
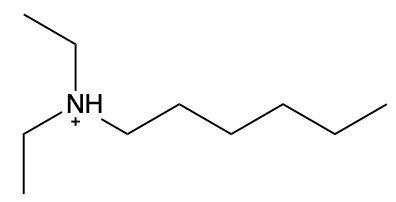
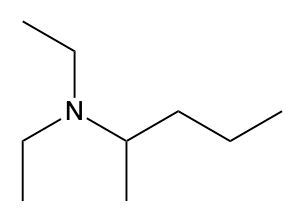
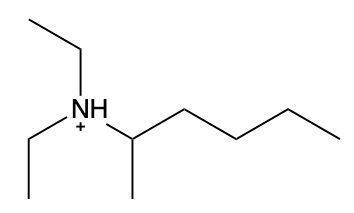
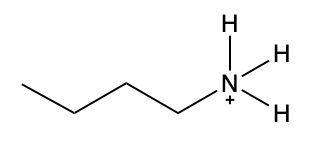
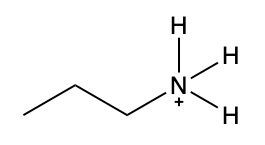
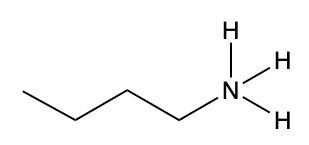
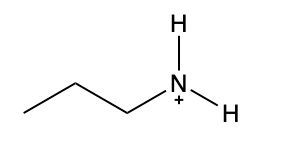
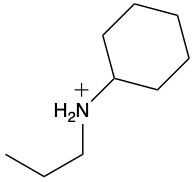
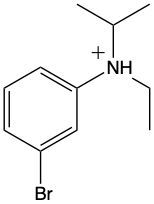
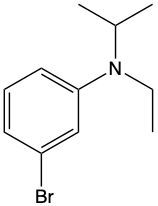
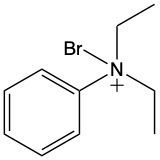
Ammonium salts, often referred to as ammonium ions, are unique compounds characterized by the presence of one or more alkyl groups attached to a positively charged nitrogen atom. The naming convention for these salts closely resembles that of amines. In the case of amines, the naming format involves stating the substituent followed by the suffix "amine." Conversely, when naming ammonium salts, the format shifts slightly to include the substituent followed by "ammonium ion."
It is important to note that while the ammonium ion can be represented as NH_4^+, this specific form is not the focus here. Instead, the emphasis is on ammonium salts that are connected to carbon atoms. Therefore, when naming these structures, one should always consider the presence of at least one alkyl group linked to the positively charged nitrogen atom, which is essential for correctly identifying and naming the compound.