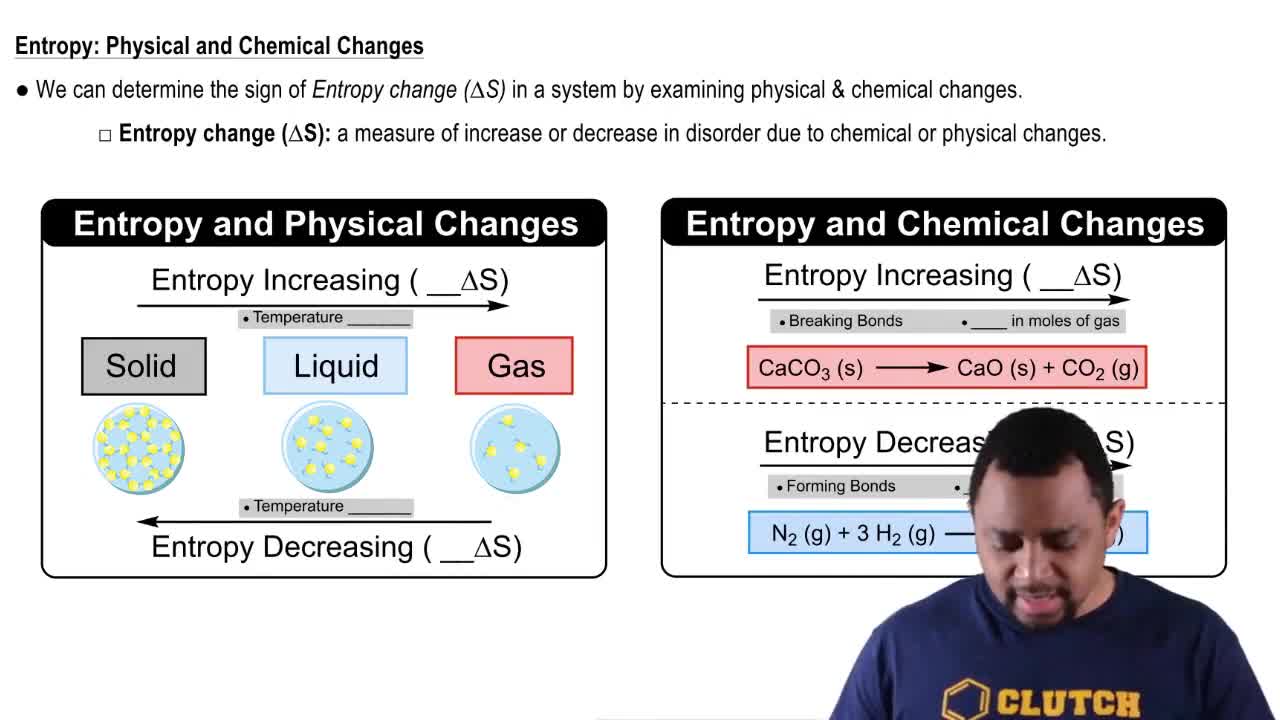
Does entropy increase or decrease in the following processes?
a. Polymeric complex carbohydrates are metabolized by the body, converted into smaller simple sugars.
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem:


 0:54m
0:54mMaster Entropy (Simplified) Concept 1 with a bite sized video explanation from Jules
Start learning