Textbook Question
Name the following compounds:
a.
b.
c.
1514
views
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem: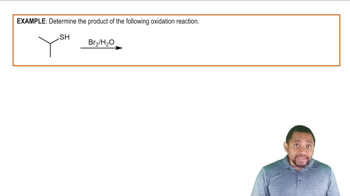



 :34m
:34mMaster Rules for Naming Ethers Concept 1 with a bite sized video explanation from Jules
Start learning