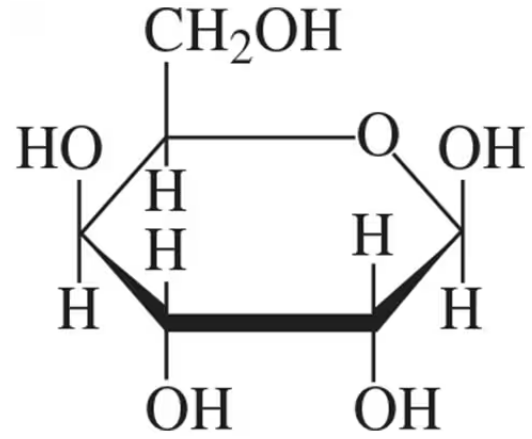
Identify each of the following as the α or ß isomer:
a.
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem:



 2:49m
2:49mMaster Cyclic Structures of Monosaccharides Concept 1 with a bite sized video explanation from Jules
Start learning