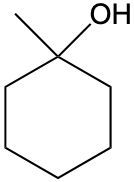
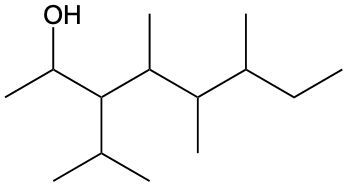
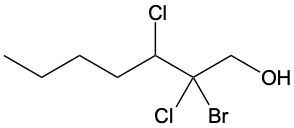
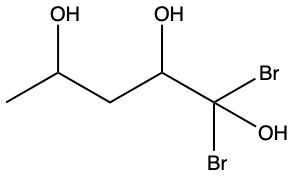
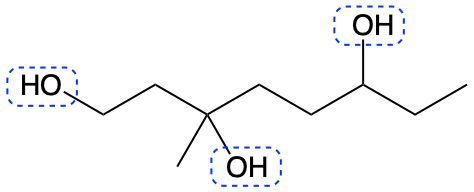
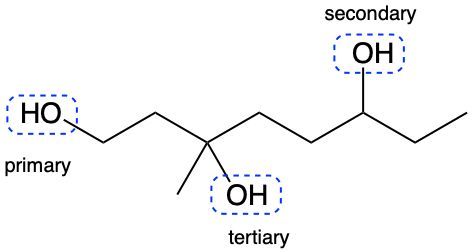
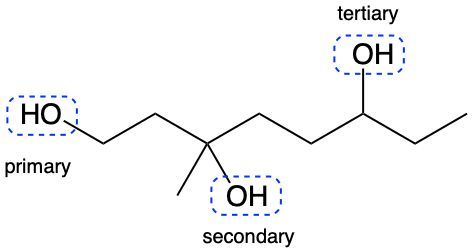
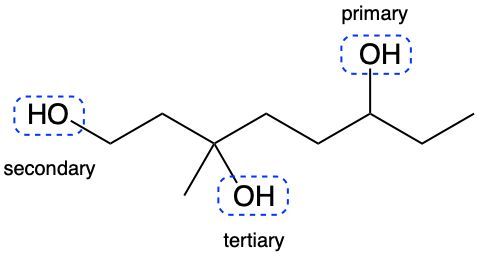
Alcohols are organic compounds characterized by the presence of a hydroxyl group (–OH) attached to a carbon atom that is sp3 hybridized. The naming of alcohols follows a set of rules that are similar to those used for alkanes, with the key difference being the addition of a modifier to the suffix. This modifier indicates the presence of the hydroxyl functional group.
The modifier for alcohols changes the suffix of the alkane name from "e" to "ol." For example, the alkane "pentane" becomes "pentanol" when a hydroxyl group is present. When naming alcohols, it is essential to specify the location of the hydroxyl group as well as any substituents or branch groups attached to the carbon chain. This is done by numbering the carbon atoms in the longest continuous chain, ensuring that the carbon with the hydroxyl group receives the lowest possible number.
In summary, when naming alcohols, remember to identify the parent alkane, modify the suffix to "ol," and indicate the positions of the hydroxyl group and any substituents. This systematic approach allows for clear and accurate communication of the structure of alcohols.