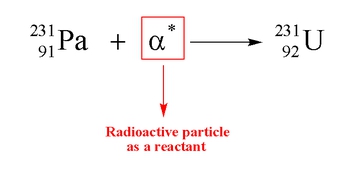
Nuclear reactions involve processes that occur within unstable atomic nuclei, fundamentally altering the number of protons in an atom. In a typical atom, electrons orbit a nucleus composed of protons, which carry a positive charge, and neutrons, which are neutral. The essence of nuclear reactions lies in their ability to change the atomic number of an element, which is defined by the number of protons it contains. This is particularly common in large, radioactive elements.
Unlike conventional chemical reactions, where the identities of the reactants remain unchanged, nuclear reactions can transform one element into another entirely different element. For instance, in a chemical reaction, we might combine hydrogen gas (H2) and nitrogen gas (N2) to produce ammonia (NH3), maintaining the identities of the original elements. However, in nuclear reactions, the alteration of protons leads to the formation of new elements. For example, a nuclear reaction could convert calcium-40 (Ca-40) into argon (Ar) by changing its proton count.
This transformation underscores the significance of the atomic number, as it directly determines the identity of an element. Each element possesses a unique atomic number, and any change in the number of protons results in the creation of a different element. Thus, nuclear reactions are characterized by their ability to fundamentally alter the nature of matter at the atomic level.