What kind of reaction does each of these enzymes catalyze?
b. A transmethylase
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem:
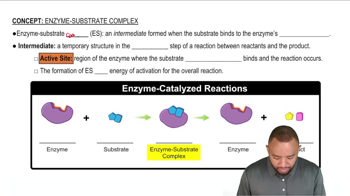

 :39m
:39mMaster Common Naming Concept 1 with a bite sized video explanation from Jules
Start learning