Textbook Question
Aldosterone is a key steroid involved in controlling the sodium–potassium balance in the body. Identify the functional groups in aldosterone.
756
views

 Verified step by step guidance
Verified step by step guidance

Aldosterone is a key steroid involved in controlling the sodium–potassium balance in the body. Identify the functional groups in aldosterone.
The compound carvone is responsible for the odor of spearmint. Identify the functional groups in carvone.
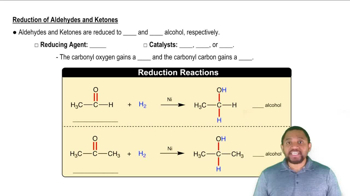
Can the alcohol (CH3)3COH be formed by the reduction of an aldehyde or ketone? Why or why not?
Draw the structural formulas of the following compounds:
c. 2-Methoxy-2-methylpropane
The liquids 1-butanol and butanal have similar molar masses. Which is expected to have the higher boiling point? Explain your choices.
Draw all the ketones you can with a chemical formula of C8H16O whose longest chain is eight carbons. Name each using both its IUPAC and common name.