Binary molecular compounds are formed from two different nonmetal elements. Common examples include water (H2O), which consists of hydrogen and oxygen, and nitrogen oxides, such as NO (nitric oxide), where nitrogen and oxygen combine. These compounds are characterized by their ability to form in various proportions, necessitating the use of numerical prefixes to indicate the number of atoms of each element present in the compound.
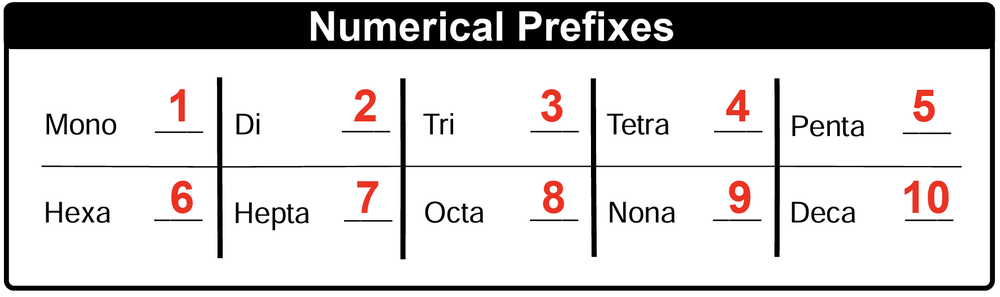
The numerical prefixes used in naming these compounds are as follows: mono- for 1, di- for 2, tri- for 3, tetra- for 4, penta- for 5, hexa- for 6, hepta- for 7, octa- for 8, nona- for 9, and deca- for 10. These prefixes are essential for accurately conveying the composition of the compound, especially when multiple combinations of the same elements can occur.
In summary, binary molecular compounds are covalent compounds made up of two different nonmetals, and the use of numerical prefixes is crucial for their proper naming and understanding of their molecular structure.