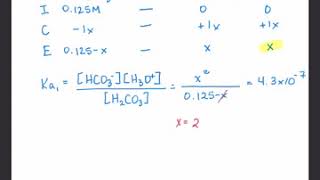17. Acid and Base Equilibrium
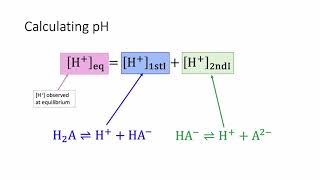
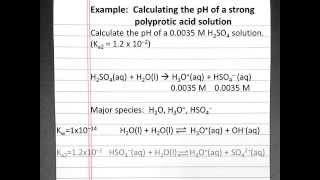
Diprotic Acids and Bases
Problem 84
Textbook Question
Textbook QuestionRainwater is acidic because CO21g2 dissolves in the water, creating carbonic acid, H2CO3. If the rainwater is too acidic, it will react with limestone and seashells (which are principally made of calcium carbonate, CaCO3). Calculate the concentrations of carbonic acid, bicarbonate ion 1HCO3-2 and carbonate ion 1CO32 - 2 that are in a raindrop that has a pH of 5.60, assuming that the sum of all three species in the raindrop is 1.0 * 10-5 M.
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
9mPlay a video:
673
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 9 videos