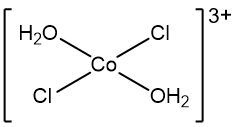
A complex ion is defined as a charged adduct that features a transition metal cation at its center, which is covalently bonded to one or more ligands. The term "ion" indicates that the complex carries an overall charge, which can be either positive or negative. While it is possible for complex ions to include main group elements, the focus here is primarily on those involving transition metals.
One key characteristic of complex ions is that they are always represented within brackets in chemical formulas. For instance, consider the complex ion formed by a copper(III) ion and four ammonia ligands. In this example, the copper ion has a charge of +3, while the ammonia ligands are neutral, contributing no charge. Therefore, the overall charge of this complex ion is +3, resulting from the sum of the charges of the metal cation and the ligands.
To summarize, a complex ion is an adduct formed by the combination of a metal cation and a specific number of ligands, and it is crucial to recognize its representation in chemical formulas, particularly by the brackets that denote its structure.