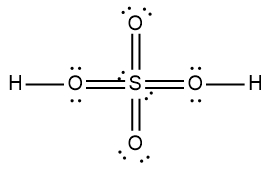
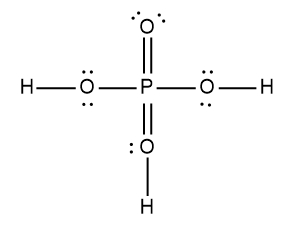
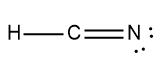In chemistry, acids are typically defined as covalent compounds that primarily consist of nonmetals and start with a hydrogen ion, often referred to as the hydronium ion. Common examples of acids include hydrobromic acid (HBr), nitric acid (HNO3), perchloric acid (HClO4), sulfuric acid (H2SO4), and phosphoric acid (H3PO4). Each of these acids is characterized by their covalent nature and the presence of hydrogen at the beginning of their molecular structure.
However, there are exceptions in the classification of acids. A notable example is acetic acid, which can be represented in two forms: as a covalent compound starting with hydrogen or as CH3COOH, which is its acidic form. This highlights the versatility in how acids can be expressed chemically.
When acids dissolve in water, they dissociate into hydrogen ions (H+) and corresponding anions. For instance, hydrobromic acid (HBr) dissociates into H+ and Br- ions. This property of acids to break apart in solution is a key characteristic, emphasizing their role as soluble covalent compounds that release hydrogen ions when dissolved.