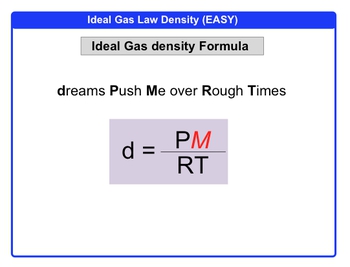
Density is a fundamental concept in science that quantifies how much mass is contained in a given volume. The formula for density can be expressed as:
\( \text{Density} = \frac{\text{Mass}}{\text{Volume}} \)
In this equation, mass is measured in grams (g), and volume is typically measured in liters (L) when dealing with gases. This distinction is important because gases have significantly lower densities compared to solids and liquids. Therefore, the appropriate unit for gas density is grams per liter (g/L), rather than grams per milliliter (g/mL) or grams per cubic centimeter (g/cm³).
To summarize, when calculating the density of a gas, ensure that you use the mass in grams and the volume in liters, reflecting the unique properties of gases in comparison to other states of matter.