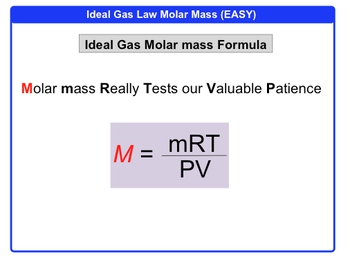
Understanding molar mass is essential in the study of gases, particularly when applying the ideal gas law. Molar mass is defined as the mass of a substance divided by the amount of that substance, typically expressed in grams per mole (g/mol). In this context, we denote molar mass with the symbol M, the mass of the gas in grams as m, and the amount of gas in moles as n.
The relationship can be summarized by the equation:
M = \frac{m}{n}
Where M is the molar mass, m is the mass of the gas, and n is the number of moles. This equation allows us to calculate the molar mass of a gas when we know its mass and the number of moles present.
By integrating this concept with the ideal gas law, which is expressed as:
PV = nRT
where P is pressure, V is volume, n is the number of moles, R is the ideal gas constant, and T is temperature in Kelvin, we can derive the molar mass of a gas under specific conditions. This application of the ideal gas law provides a practical method for determining the molar mass, enhancing our understanding of gas behavior in various scenarios.