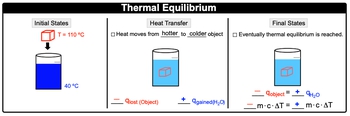
Thermal equilibrium occurs when two substances in contact reach the same temperature, resulting in no net exchange of thermal energy, as described by the first law of thermodynamics. For instance, consider a heated metal cube at 110 degrees Celsius placed in water at 40 degrees Celsius. Heat naturally transfers from the hotter object (the cube) to the colder one (the water). In this scenario, the cube loses heat, represented as a negative heat transfer (q), while the water gains heat, indicated as a positive q. This process continues until both the cube and the water reach the same temperature, signifying thermal equilibrium.
At thermal equilibrium, the heat lost by the hotter object equals the heat gained by the colder object, expressed mathematically as:
\[-q_{\text{cube}} = q_{\text{water}}\]
In terms of mass and specific heat capacity, this relationship can be represented as:
\[-m_{\text{cube}}c_{\text{cube}}(T_f - T_{\text{cube}}) = m_{\text{water}}c_{\text{water}}(T_f - T_{\text{water}})\]
where \(T_f\) is the final equilibrium temperature.
In ideal conditions, heat transfer occurs solely between the heated object and the solvent. However, in non-ideal situations, the calorimeter (or container) may also absorb some heat. In such cases, the equation expands to account for the calorimeter's heat absorption:
\[-q_{\text{object}} = q_{\text{water}} + q_{\text{calorimeter}}\]
Thus, when solving thermal equilibrium problems, if only two substances are involved, the heat lost by the hotter object equals the heat gained by the colder object. If the calorimeter is included, its heat absorption must also be considered to accurately reflect the total heat transfer in the system.