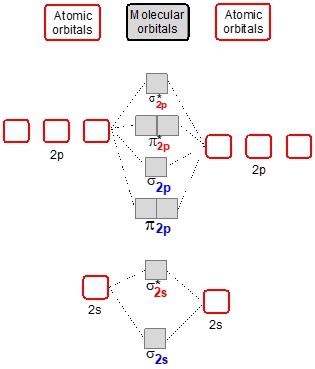
A heteronuclear diatomic molecule consists of two different elements bonded together, where the less electronegative element influences the molecular orbital used. Electronegativity, which increases towards the top right of the periodic table, is crucial in determining the energy levels of atomic orbitals. For instance, fluorine, with the highest electronegativity of 4.0, exemplifies this trend.
In molecular orbital diagrams, the more electronegative element has atomic orbitals that are lower in energy. For example, when comparing carbon and nitrogen, nitrogen's higher electronegativity results in its atomic orbitals being positioned lower in energy than those of carbon. This pattern is consistent across other pairs, such as oxygen and neon, where the 2s atomic orbital of the less electronegative element is higher in energy.
Understanding these energy differences is essential when analyzing molecular orbital diagrams. The higher the electronegativity, the closer the electrons are to the nucleus, indicating a stronger attraction and tighter binding. This phenomenon is particularly evident in elements with lower shell numbers, like fluorine, where the electrons are more tightly bound due to increased electronegativity.
In summary, electronegativity plays a pivotal role in determining the energy levels of atomic orbitals in heteronuclear diatomic molecules, influencing both the molecular orbital diagrams and the behavior of electrons within these structures.