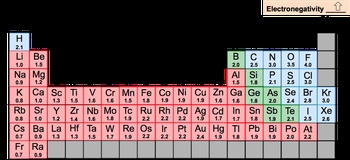
Electronegativity, often abbreviated as EN, quantifies an element's ability to attract electrons. It is essential to distinguish electronegativity from electron affinity; while electronegativity measures attraction, electron affinity refers to the energy released when an electron is added to an element. This concept was notably advanced by American chemist Linus Pauling in 1932, marking a significant contribution to general chemistry.
The periodic trend indicates that electronegativity increases as one moves from left to right across a period and up a group in the periodic table. This trend leads to higher electronegativity values in the top right corner of the table. However, exceptions exist, particularly among transition metals, due to the complexities of their d and f orbitals, which can disrupt expected trends. Additionally, noble gases typically lack electronegativity values because they do not tend to attract electrons.
It is also noteworthy that many elements in the bottom row of the periodic table are not assigned electronegativity values due to their large size and instability. Among all elements, fluorine is recognized as the most electronegative, while francium is the least electronegative. Understanding these distinctions is crucial for grasping the behavior of elements in chemical reactions, particularly in terms of their electron interactions.