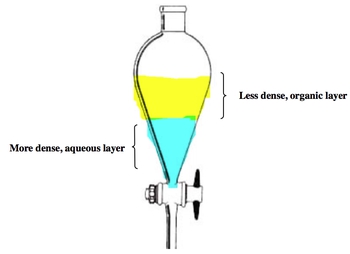
Extraction is a crucial technique in chemistry that involves the separation of a solid from a liquid, often through selective precipitation in a new solvent. A common method for performing extraction is using a separatory funnel, which allows for the separation of two immiscible liquids based on their densities. In this context, the denser liquid, often water, forms the aqueous phase at the bottom, while the less dense organic layer sits above it.
In a typical extraction scenario, you might have two compounds in the organic phase, such as ammonia (NH3) and acetic acid (CH3COOH). Acid-base reactions are frequently employed in these extractions, where the pH of the system is adjusted by adding strong or weak acids or bases. The solubility of the compounds in either the aqueous or organic layers is influenced by their pKa values. When an acid or base is added, it can change the ionic state of a compound, thereby affecting its solubility.
For instance, if you want to extract acetic acid from the organic layer, you can add a strong base. This base will deprotonate acetic acid, converting it into the acetate ion (CH3COO-), which is polar and thus more soluble in the aqueous layer. Once the acetate ion is in the aqueous layer, it can be collected by draining the layer into a beaker.
To convert the acetate ion back into solid acetic acid, you can introduce an acid to the aqueous solution. This will provide H+ ions, converting the acetate back to acetic acid, which is neutral and will precipitate out of the solution. The solid can then be separated from the liquid phase.
To ensure that all acetic acid has been removed from the organic layer, pH strips can be used to test the acidity of the organic phase. If the pH is basic, it indicates that all acetic acid has been neutralized. It is also important to note that mixing acids and bases can generate gas, leading to pressure buildup in the separatory funnel. To safely release this pressure, the funnel should be inverted with the opening pointed upwards, allowing gas to escape while mixing the contents thoroughly.
This method of extraction is not only effective for separating two compounds but can also be adapted for more complex mixtures, allowing for the selective separation of multiple components based on their acid-base properties.