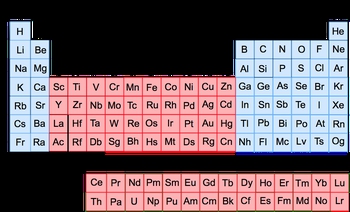
The periodic table organizes elements into rows, known as periods, and columns, referred to as groups. Within these groups, elements can be classified as either transition metals or representative elements. Transition metals are located in groups 3 to 12 and are characterized by their ability to exhibit varying positive charges, which can range from +1 to +7 depending on the element they bond with. For instance, manganese (Mn) is a transition metal that can have multiple oxidation states.
Additionally, there are inner transition metals, which are found in the two rows separated from the main body of the periodic table, specifically between lanthanum (La) and hafnium (Hf), and between actinium (Ac) and rutherfordium (Rf). These elements are still classified as transition metals but are distinguished due to their unique placement.
Transition metals are also referred to as group B elements. This classification allows for further labeling of these groups: group 3 can be labeled as 3B, group 4 as 4B, and so forth. Notably, groups 8, 9, and 10 are collectively referred to as 8B, while group 11 is designated as 1B and group 12 as 2B. Understanding this classification is essential, even if the reasoning behind the naming conventions is complex.
On the other hand, representative elements encompass all elements not found in groups 3 to 12, specifically those in groups 1, 2, and 13 to 18. These elements are sometimes called group A or main group elements. For example, group 1 is labeled as 1A, group 2 as 2A, and this continues up to group 8A. Recognizing these classifications helps in understanding the properties and behaviors of different elements within the periodic table.
In summary, the periodic table can be further dissected into transition metals (group B elements) and representative elements (group A or main group elements), each with distinct characteristics and classifications that aid in the study of chemistry.