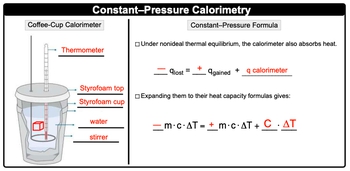
A calorimeter is an insulated container designed to minimize heat loss, typically filled with a liquid that has a specific heat capacity. When a heated object is introduced into the liquid, both the liquid and the calorimeter absorb the heat released by the object. The standard heat capacity of the calorimeter, denoted as \( C \), represents the amount of heat required to change its temperature.
The formula for standard heat capacity can be expressed as:
\( C = \frac{q}{\Delta T} \)
In this equation, \( q \) represents the heat absorbed, while \( \Delta T \) is the change in temperature. The standard heat capacity is typically measured in joules per degree Celsius (J/°C). If temperature is provided in Kelvin, it is essential to convert it accordingly, ensuring that the units are consistent throughout the calculation.
It is important to note that \( q \) can be expressed in either joules or kilojoules, so careful attention must be paid to unit compatibility to ensure they cancel appropriately, allowing for the isolation of the desired variable.