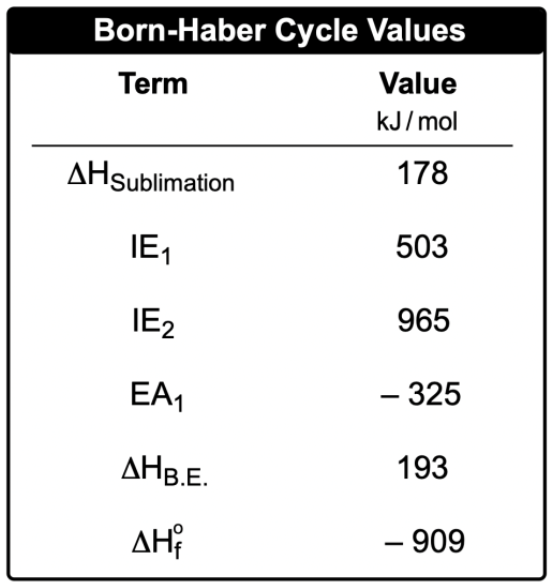
The Born-Haber cycle is a systematic approach to understanding the formation of an ionic compound from its constituent elements in their standard states. This cycle outlines the necessary steps to calculate the enthalpy of formation, denoted as ΔHf°, which represents the heat change when one mole of a compound is formed from its elements. For instance, the formation of sodium chloride (NaCl) involves combining one mole of solid sodium (Na) with half a mole of chlorine gas (Cl2).
To derive the enthalpy of formation, both sodium and chlorine must first be converted into their gaseous ionic forms. The process begins with the sublimation of solid sodium, which requires energy and is quantified as the enthalpy of sublimation. This step transforms sodium from a solid to a gas. Following this, ionization energy is applied to remove an electron from the gaseous sodium, resulting in a positively charged sodium ion (Na+). The first ionization energy, denoted as IE1, is relevant here, and if multiple electrons were to be removed, subsequent ionization energies (IE2, IE3, etc.) would be considered.
Next, chlorine gas (Cl2) must be dissociated into individual chlorine atoms. This process, known as the enthalpy of dissociation, involves breaking the bond between the two chlorine atoms. Once separated, an electron is added to one of the chlorine atoms, a process quantified by electron affinity (EA). The first electron affinity, EA1, is used for this step, with additional electron affinities (EA2, EA3, etc.) applicable if more electrons are added.
After obtaining both sodium and chlorine in their ionic gaseous forms, they can combine to form solid sodium chloride. This final step is associated with lattice energy, which is the energy released when gaseous ions come together to form a solid ionic lattice. The entire Born-Haber cycle thus consists of five fundamental steps: enthalpy of sublimation, ionization energy, enthalpy of dissociation, electron affinity, and lattice energy. Understanding this cycle is crucial for justifying how sodium and chlorine react to form sodium chloride, illustrating the intricate energy changes involved in ionic compound formation.