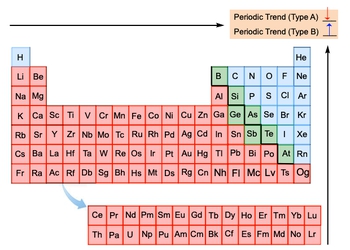
Periodic trends in the periodic table can be categorized into two main groups based on their behavior as you move towards the top right corner. The first group, referred to as Type A trends, includes metallic character and atomic radius. Both of these trends exhibit a decrease as you progress towards the top right corner of the periodic table. This means that elements become less metallic and their atomic size diminishes in this direction.
The second group, known as Type B trends, encompasses ionization energy, electron affinity, electronegativity, and effective nuclear charge. These trends show an increase as you move towards the top right corner. This indicates that elements require more energy to remove an electron, have a greater tendency to attract electrons, and possess a stronger effective nuclear charge in this region.
It is important to note that ionic radius does not fit neatly into either category, as it is influenced by the number of electrons and their arrangement. Understanding these trends allows for a comprehensive overview of how elements behave in relation to one another on the periodic table, facilitating easier predictions of their chemical properties.