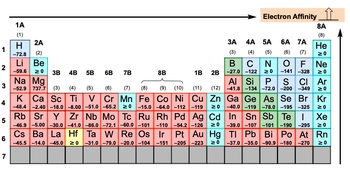
Electron affinity, denoted as \( e_a \), refers to the energy released when an electron is added to a gaseous atom or ion, typically measured in kilojoules. This process is exothermic, meaning it releases energy. For instance, when an electron is added to a neutral carbon atom, it transforms into a negatively charged ion, represented as \( \text{C}^- \) in the gaseous state. The release of energy during this process results in a negative value for electron affinity.
The magnitude of the electron affinity value indicates the extent of the exothermic reaction; the more negative the value, the more energy is released, signifying a stronger tendency for the atom to accept an electron. However, there are exceptions to this trend, particularly when examining the periodic table. If the electron affinity is equal to or greater than zero, it suggests that the element is less inclined to accept an electron, as doing so would disrupt its stability.
Understanding electron affinity is crucial for predicting how elements interact with electrons, which plays a significant role in chemical bonding and reactivity.