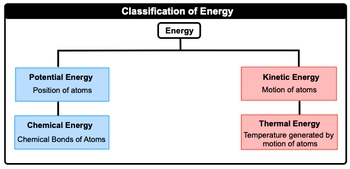
Thermal chemistry explores the relationship between matter and energy during chemical reactions and physical changes. At its core, energy is defined as the capacity to perform work or generate heat. In this context, energy can be categorized into two primary forms: potential energy and kinetic energy.
Potential energy refers to the energy associated with the position of atoms, while kinetic energy relates to the energy of motion. These two forms can be further subdivided. Potential energy can be linked to chemical energy, which is the energy stored in the bonds between atoms. On the other hand, kinetic energy can be associated with thermal energy, which arises from the temperature generated by the motion of atoms.
In summary, the key types of energy relevant to thermal chemistry include:
- Potential Energy: Energy of position, including chemical energy.
- Kinetic Energy: Energy of motion, including thermal energy.
Understanding these energy forms is crucial as they play a significant role in the study of thermal chemistry, where the focus is primarily on how these energies interact during chemical processes.



 3 students found this helpful
3 students found this helpful