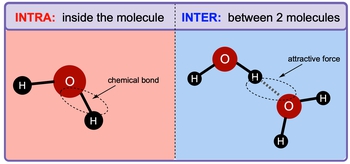
Intermolecular forces and intramolecular forces are two fundamental types of electrostatic forces that play crucial roles in the behavior of substances. Intramolecular forces are the attractive forces that exist within a molecule, bonding atoms together and significantly influencing the chemical properties of that molecule. These forces are categorized into two main types: ionic and covalent bonds. It is important to note that intramolecular forces are generally stronger than intermolecular forces.
In contrast, intermolecular forces are the attractive forces that occur between separate molecules. These forces are essential for holding together the molecules in liquids and solids, thereby influencing their physical properties. For example, in water (H2O), the intramolecular force is the bond between the oxygen atom and the hydrogen atoms, which is a strong covalent bond. On the other hand, the intermolecular forces are the attractions between different water molecules.
The concept of electronegativity is key to understanding these intermolecular attractions. In water, oxygen is more electronegative than hydrogen, resulting in a partial negative charge on the oxygen atom and a partial positive charge on the hydrogen atoms. This difference in polarity leads to the attraction between water molecules, which is represented by a dotted line in diagrams, indicating that it is an attractive force rather than a true bond.
In summary, while intramolecular forces create the bonds that define the structure of a molecule, intermolecular forces govern the interactions between molecules, affecting the physical state and properties of substances.