Combustion analysis is a vital analytical technique used to determine the empirical formula of a compound. This process involves a specialized combustion apparatus designed to facilitate the combustion of a sample, allowing for the identification of its elemental composition. The apparatus typically consists of multiple chambers, labeled A through D, each serving a specific function in the analysis.
Initially, the sample is placed in chamber A, where it is vaporized in the presence of oxygen gas (O2), a crucial reactant in combustion reactions. Once vaporized, the sample moves to chamber B. Here, if the sample contains hydrogen, it is converted into water (H2O). Additionally, any nonmetal components of the sample are transformed into gaseous products. For instance, if carbon is present, it will typically be converted into carbon dioxide (CO2); if nitrogen is present, it becomes nitrogen dioxide (NO2); sulfur converts to sulfur dioxide (SO2); and halogens form diatomic molecules.
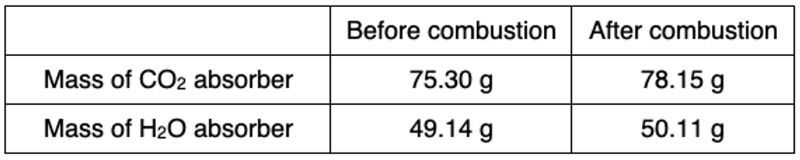
In chamber C, the water produced is collected, while the gases generated from the nonmetals are also captured. The identity of these gases depends on the nonmetal present in the original sample. After the combustion process, any excess oxygen that remains may also be released, particularly in scenarios where the combustion occurs in an oxygen-rich environment.
Understanding the function of each chamber in the combustion apparatus is essential for interpreting the results of combustion analysis. This method not only reveals the empirical formula of the compound but also provides insights into its elemental composition, making it a fundamental technique in analytical chemistry.