14. Solutions
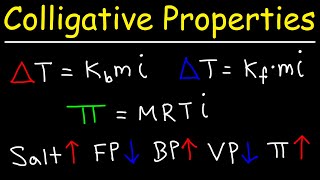
Vapor Pressure Lowering (Raoult's Law)
Problem 74
Textbook Question
Textbook QuestionA solution contains a mixture of pentane and hexane at room temperature. The solution has a vapor pressure of 258 torr. Pure pentane and hexane have vapor pressures of 425 torr and 151 torr, respectively, at room temperature. What is the mole fraction composition of the mixture? (Assume ideal behavior.)
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
4mPlay a video:
2894
views
1
comments
Was this helpful?
Related Videos
Related Practice
Showing 1 of 7 videos