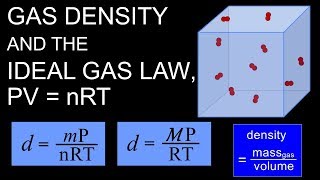
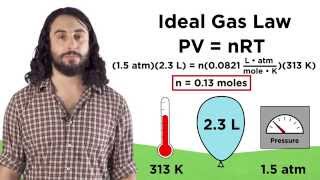
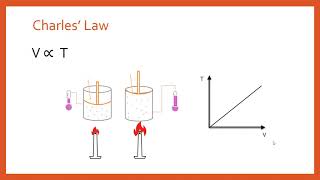
7. Gases
The Ideal Gas Law Derivations
Problem 49b
Textbook Question
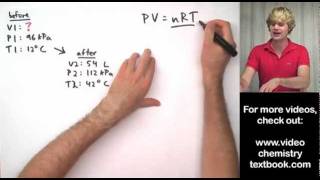
Textbook QuestionAssume that you have a cylinder with a movable piston. What would happen to the gas volume of the cylinder if you were to do the following? (b) Increase the amount of gas by one-fourth while holding the temperature and pressure constant
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
4mPlay a video:
420
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 12 videos