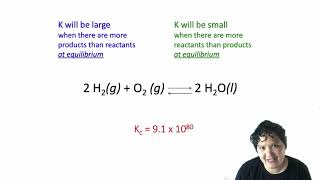16. Chemical Equilibrium
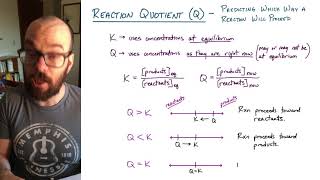
Reaction Quotient
Problem 43b
Textbook Question
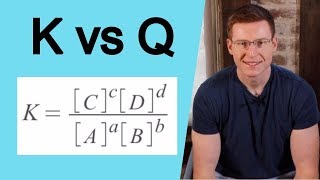
Textbook QuestionAt 100 C, the equilibrium constant for the reaction COCl21g2 Δ CO1g2 + Cl21g2 has the value Kc = 2.19 * 10-10. Are the following mixtures of COCl2, CO, and Cl2 at 100 C at equilibrium? If not, indicate the direction that the reaction must proceed to achieve equilibrium. (a) 3COCl24 = 2.00 * 10-3 M, 3CO4 = 3.3 * 10-6 M, 3Cl24 = 6.62 * 10-6 M
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
5mPlay a video:
650
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 10 videos